Usp 797 Master Formulation Record Template
June 3 2019 addition of a new requirement for master formulation records for csps prepared for more than one patient and for csps prepared from nonsterile ingredient s and compounding records for all csps.
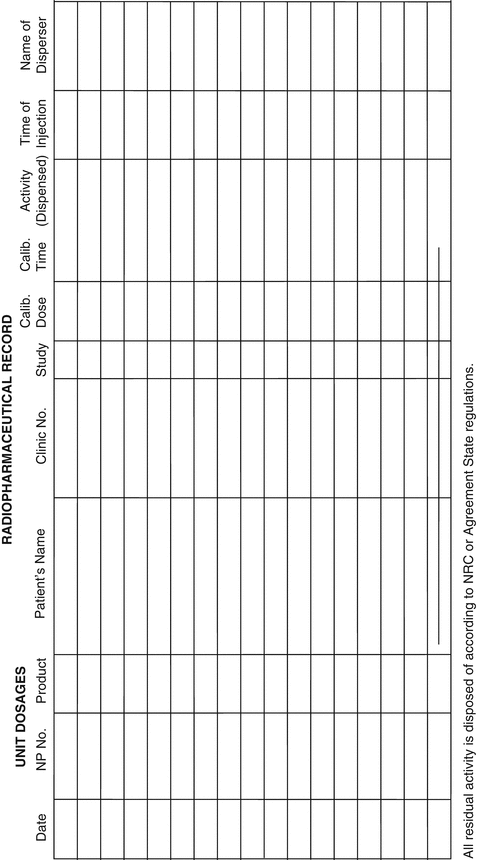
Usp 797 master formulation record template. Pharmacists are advised to follow communications from usp concerning potential updated revisions to usp chapter 797 anticipated to come into effect on. The following is a best practice recommendation on the elements of a master formulation record. The united states pharmacopeia usp is an agency that requires pharmacists to create a compound record and a mater formulation record for any compounded drugs. A master formulation record should be created before compounding a preparation for the first time. A prescription medication order or label may serve as the compounding record provided that it.
Usp 42 nf 37 second supplement. In accordance with usp s rules and procedures of the council of experts rules and except as provided in section 7 02 accelerated revision processes usp publishes proposed revisions to the united states pharmacopeia and the national formulary. In addition a compounding record should be completed each time a preparation is compounded. Assigned name and strength use dosage form recorded by formula quantity container size and type storage requirements bud recorded date compounding equipment and techniques revision date ingredient or supply quantity. All formulations prepared in a compounding pharmacy must have.
A master formulation record. Select the best possible answer. Not sure how in depth these master formulation logs need to be and was hoping any of y all could share what you use. Either a master formulation record or a compounding. Master formulation record formula number.
A master formulation record is recommended when performing batch or high risk compounding. Dosage forms 1151 pharmaceutical calculations in prescrip this record shall be followed each time that prepara tion compounding 1160 quality assurance in pharmaceuti tion. Ingredients used in the formulation have their expected identity quality and purity. A master formulation record documents information particular to the lot numbers of ingredients regarding specific batch preparations. Does anyone have a master formulation record compounding log template you d be willing to share that is in compliance with the new usp 795 797 regs on nonsterile and sterile compounding.
A master formulation record should be created compounding sterile preparations 797 pharmaceutical before compounding a preparation for the first time. June 28 2019.
free promissory note template word document free pay stub template google docs free printable late rent notice template free michigan quit claim deed template free last will and testament template nj free online residential lease agreement template free loan agreement template between family members