Software Validation Procedure Iso 13485 Template
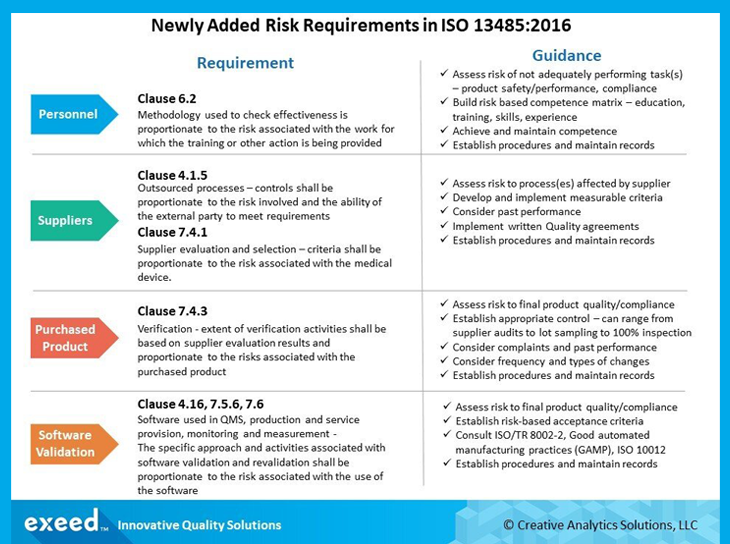
Iso 13485 2016 section 4 1 6 quality management system general requirements and 7 5 6 validation of processes for production and service provision state the following the organisation shall document procedures for the validation of the application of computer software used in the quality management system.
Software validation procedure iso 13485 template. We had an audit and the auditors pointed out that the iso 13485 2016 section 4 1 6 states that any software related to the qms should be validated before to the prior use. The document is optimized for small and medium sized organizations we believe that overly complex and lengthy documents are just overkill for you. Iso 13485 2016 standard 4 1 6 validation of software applications for the quality management system itay abuhav 06 11 2018 0 software validation is a critical tool used to ensure that the realization processes operated by software automated operations are performing as expected. The first detail to focus on is the creation of a quality procedure or sop for the evaluation and validation of software used in the quality system. Use this free diagram of iso 13485 2016 implementation process to plan implementation of production process validation.
The procedure should reference iso 13485 2016 and outline a risk based approach to evaluating current updated and new software that will be used in the quality system. The fulfillment of iso 13485 process validation requirements gives manufacturers suppliers and customers the necessary confidence to keep the business cycle running. 2016 and iso 17025 standards iso 13485 v 2016 4 1 6 document procedures for the validation of the application of computer software used in the quality management system. Ensure that the. The process validation procedures are iso 13485 2016 and fda qsr compliant.
The template documentation covers both iso 13485 2003 and fda qsr 21 cfr part 820 requirements under one quality system and is thus ideally suited for companies that must comply with both the us fda and international regulations. Iso 13485 document template. We are using this erp software collmex web based since last 5 7 years. Requirements of iso 13485. Validate such software applications prior to initial use and as appropriate after changes to such software or its application.
Use our free iso 13485 procedure template and the list of iso 13485 2016 mandatory procedures to build your medical device quality system and get certified. The iso 13485 process validation procedure bundle includes 4 procedures related to the validation of medical device processes. Imsxpress iso 13485 template documentation is part of imsxpress iso 13485 software. Includes related form s digital content instant download. This procedure will.
word cover page template free download what template is this website using what template to use for resume work breakdown structure template google sheets work breakdown structure template excel free writing a small business plan template your order has shipped email template