Medical Device Design Validation Protocol Template

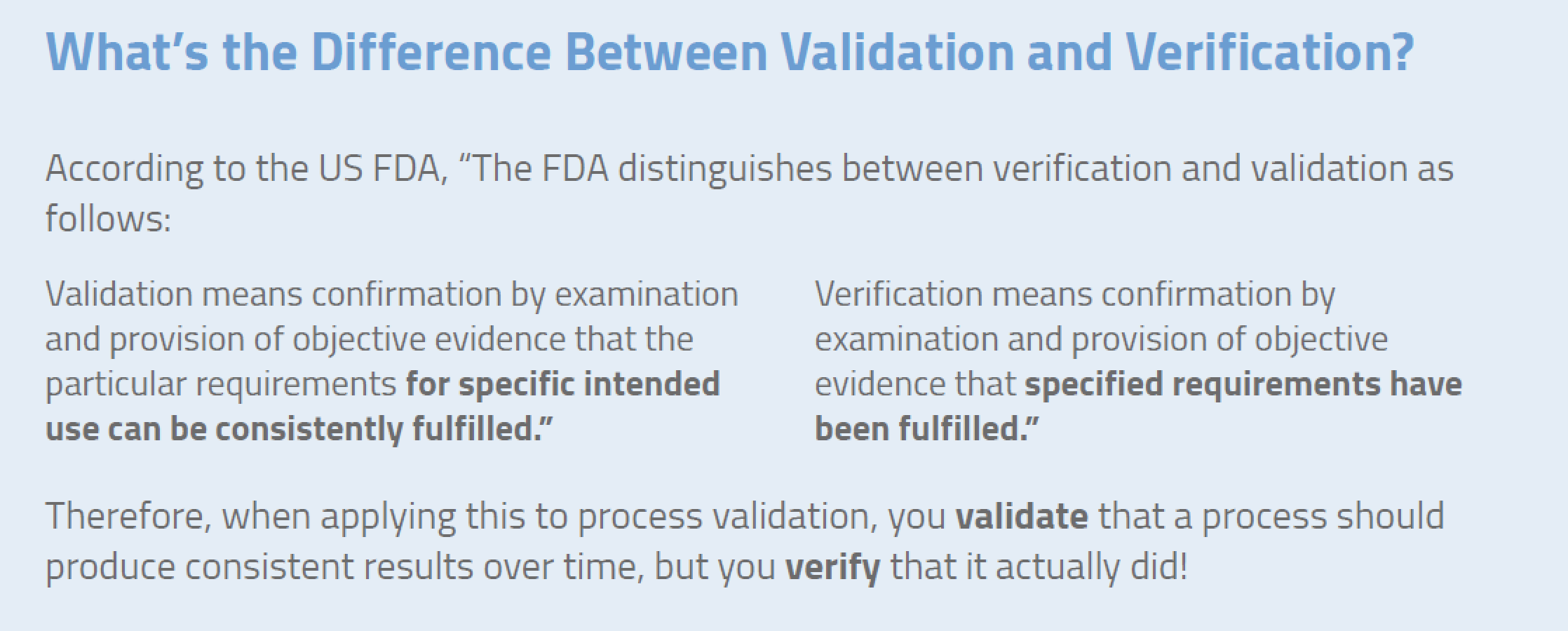
Official word from the fda 21 cfr 820 3 states that design validation is establishing by objective evidence that device specifications conform with user needs and intended use s.
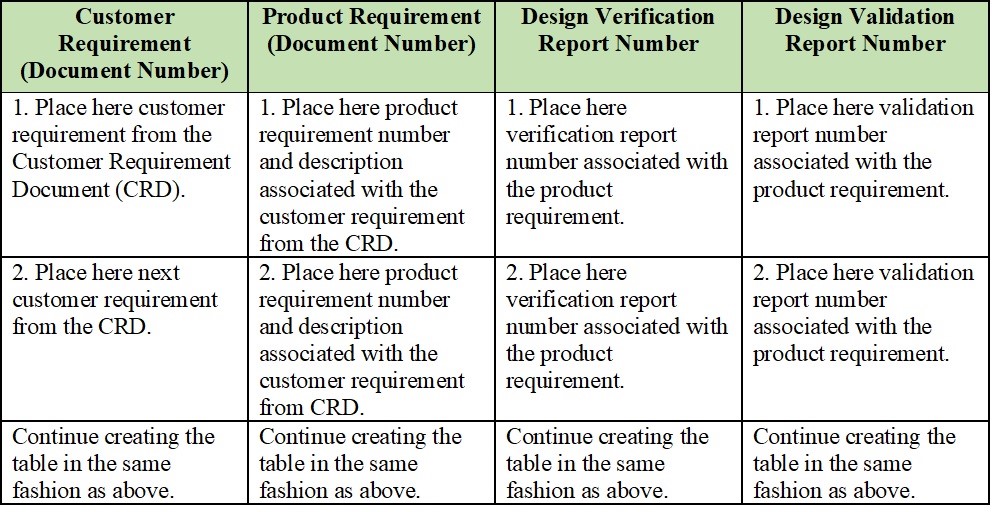
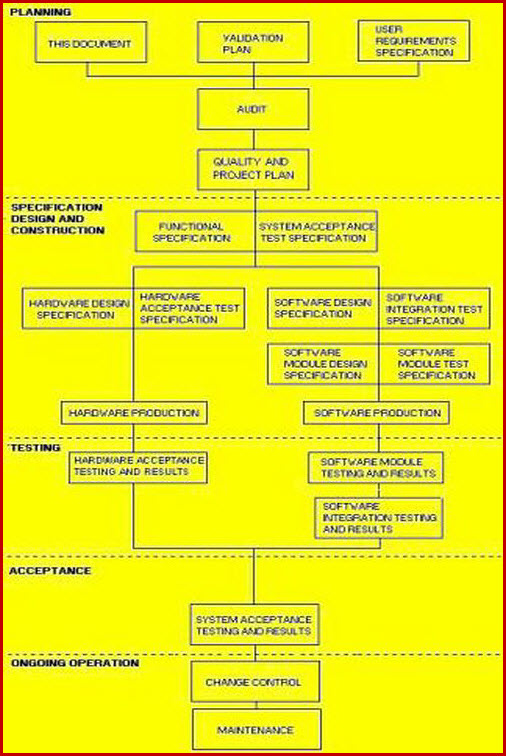
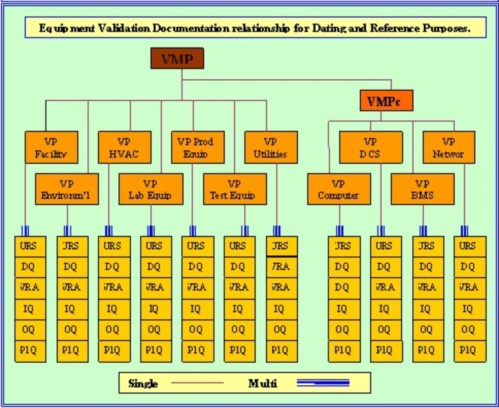
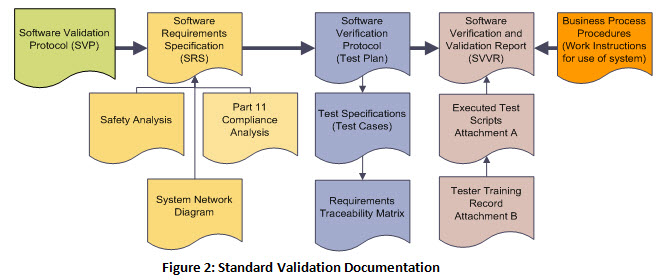
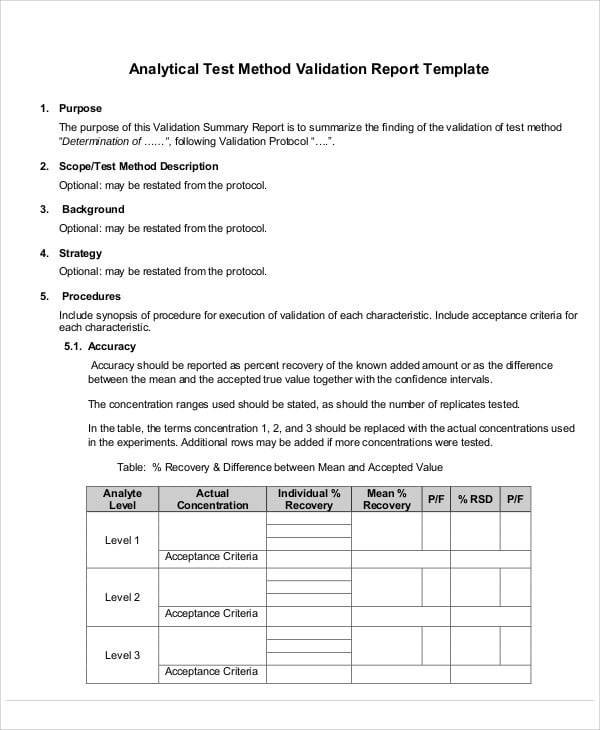
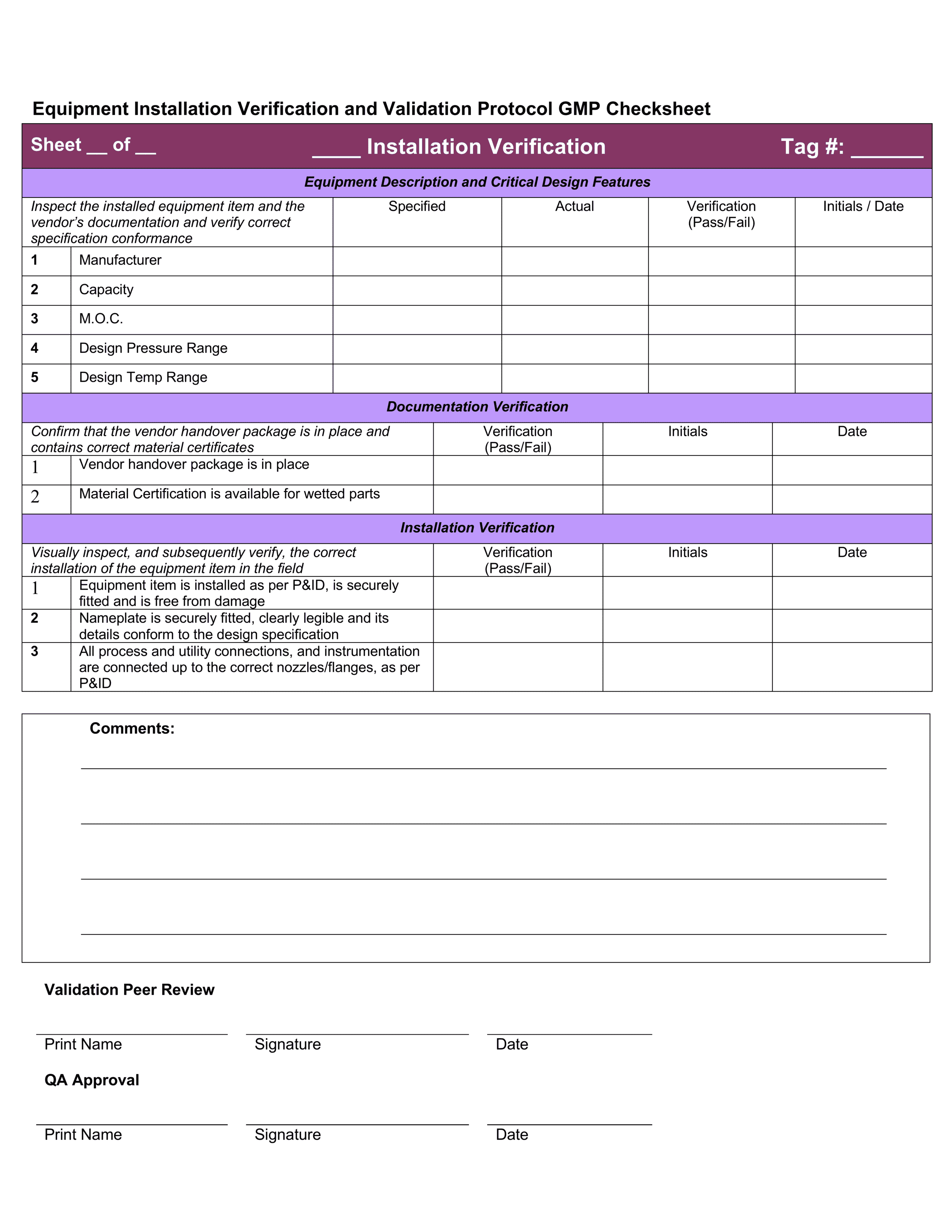
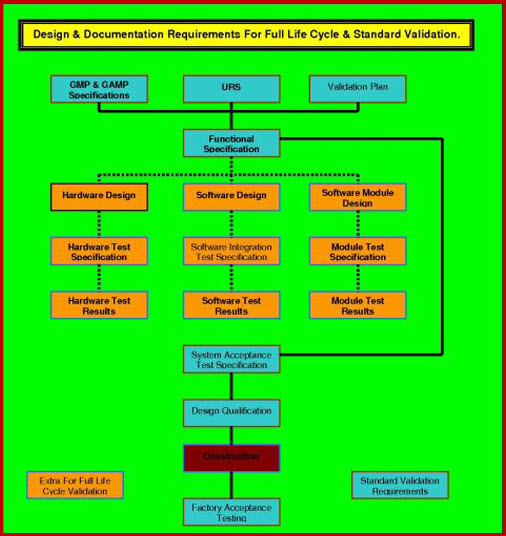
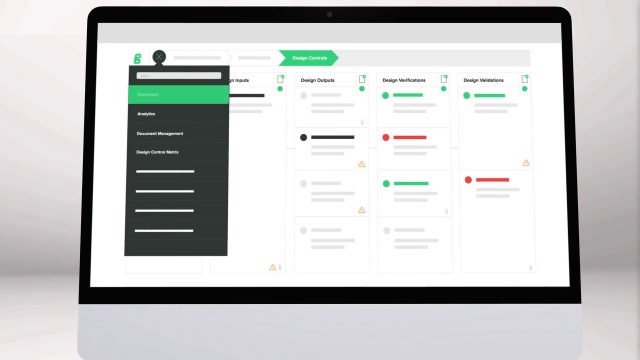
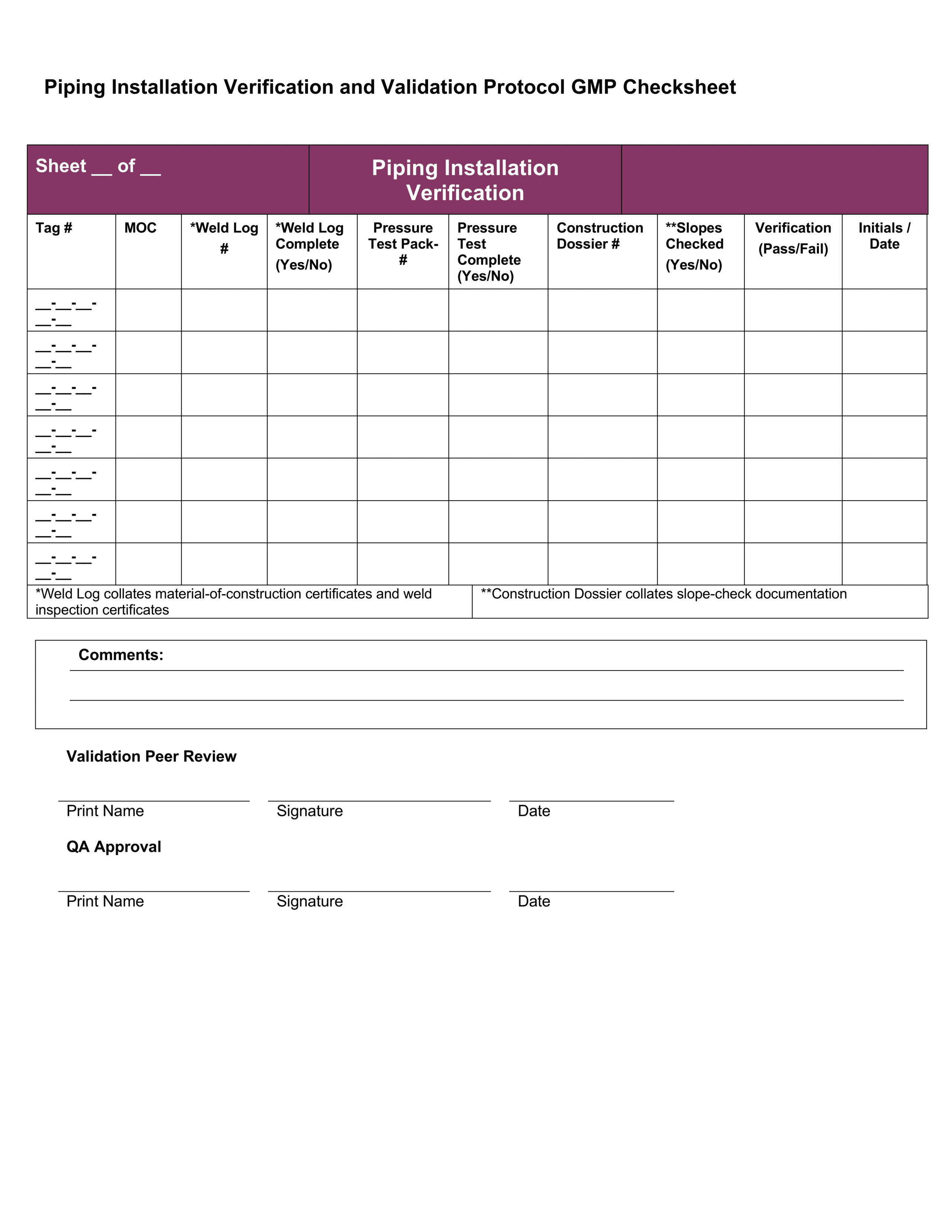
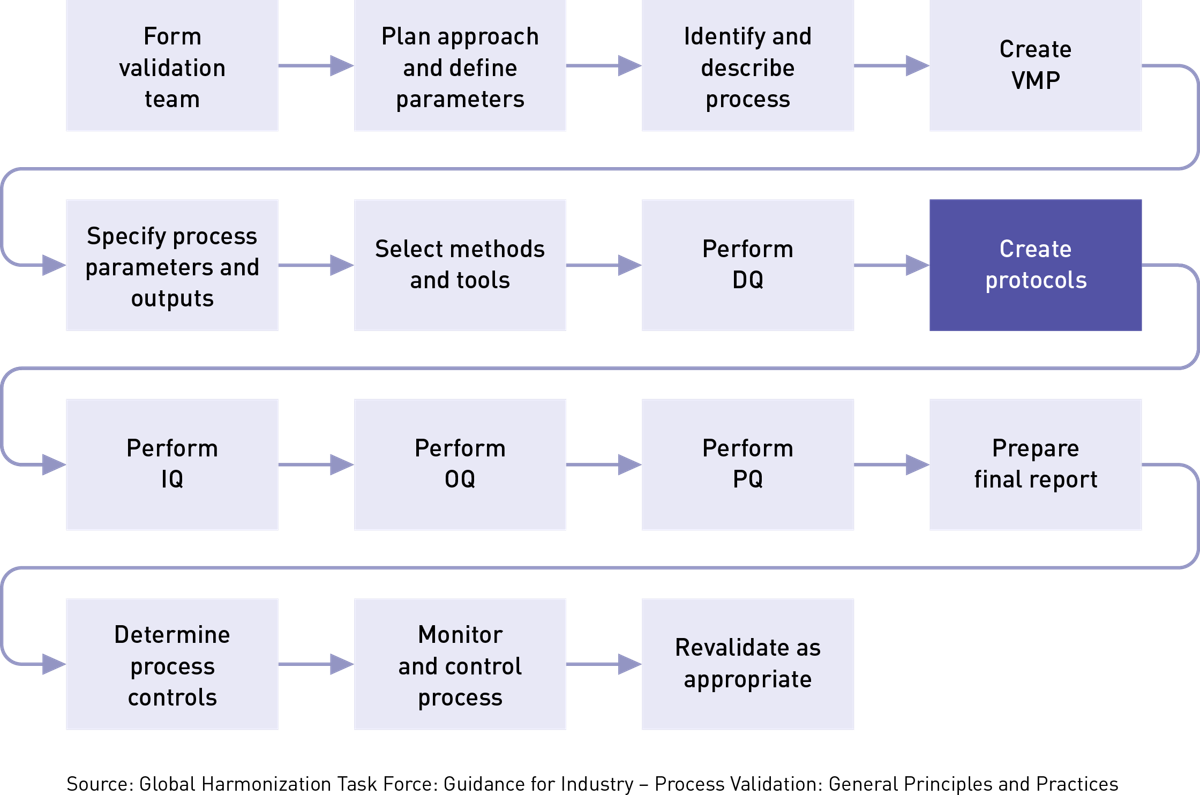
Medical device design validation protocol template. This procedure is also intended to meet the requirements of iec 62304 ed. Doing so means proving the medical device meets the user needs and intended uses. As seen below it includes facilities equipment methods and training. Step by step increase their cgmp compliance. A well written protocol will outline the correct rules policies and procedures to be followed during process validation.
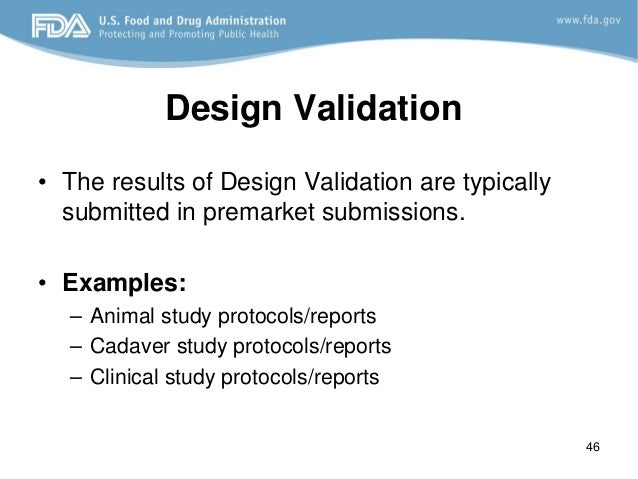
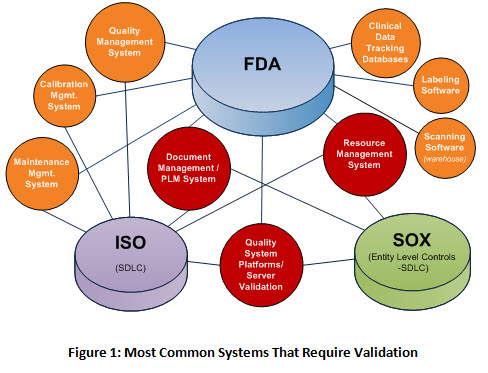
At the same time the fda medical device templates business has become highly regulated. This medical device risk analysis validation and verification course will be most valuable to medical device industry engineers engineering managers regulatory affairs professionals scientists and quality engineers needing an understanding of validation and verification v v per fda s 21 cfr part 820 30 f g iso 13485 2016 and risk. He proposes his consulting services so don t hesitate to contact him at email protected or 41799036836. Today expect medical device templates validation to perform to a high level. The growing expectation is that these subcontractors will.
Medical device validation 510k and the importance of your suppliers being fully conversant and compliant with all the regulatory obligatory requirements has become a rather burdensome load for some companies and an unacceptable load for others. 1 1 and 21 cfr 820 30 a 2 i and g. Format of a basic medical device process validation protocol. My objective is to share my knowledge and experience with the community of people working in the medical. For device validation to perform in this environment the production testing of fda medical device templates must have both compliant procedures and an excellent technical strategy.
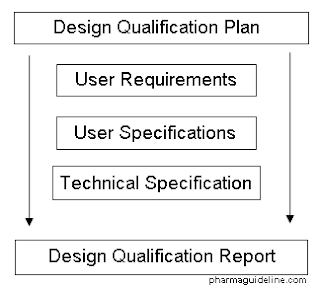
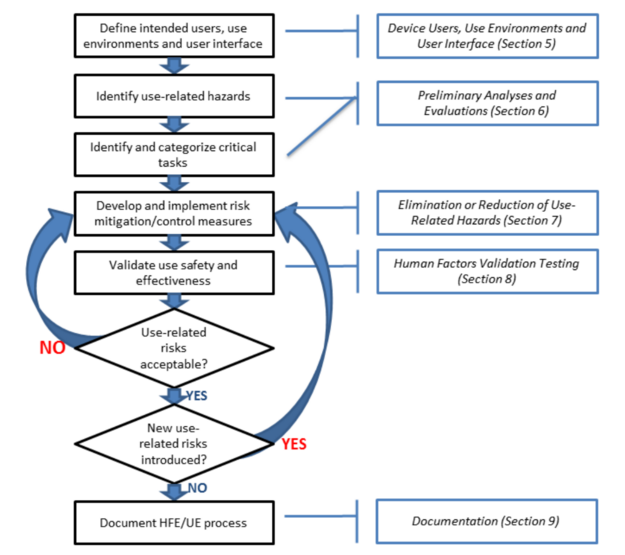
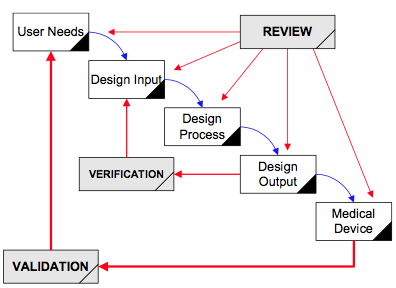
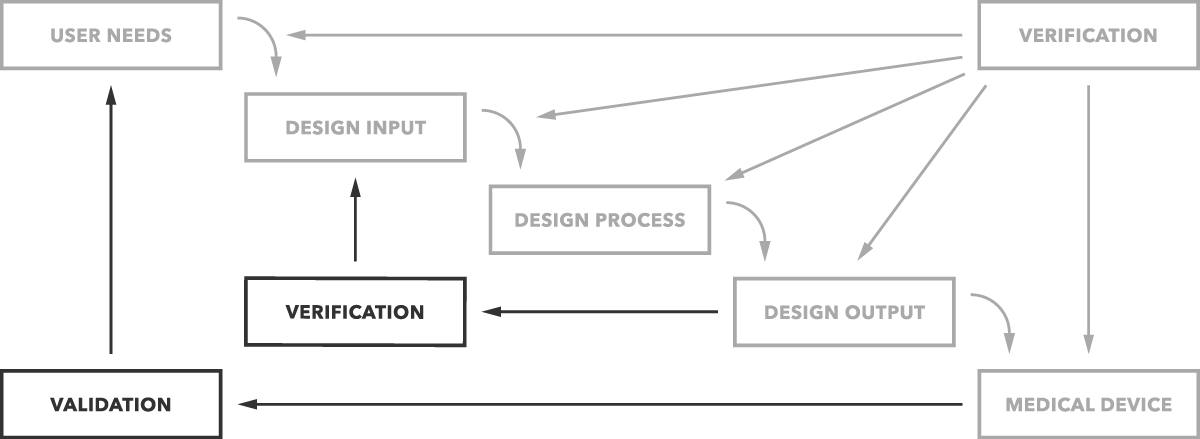
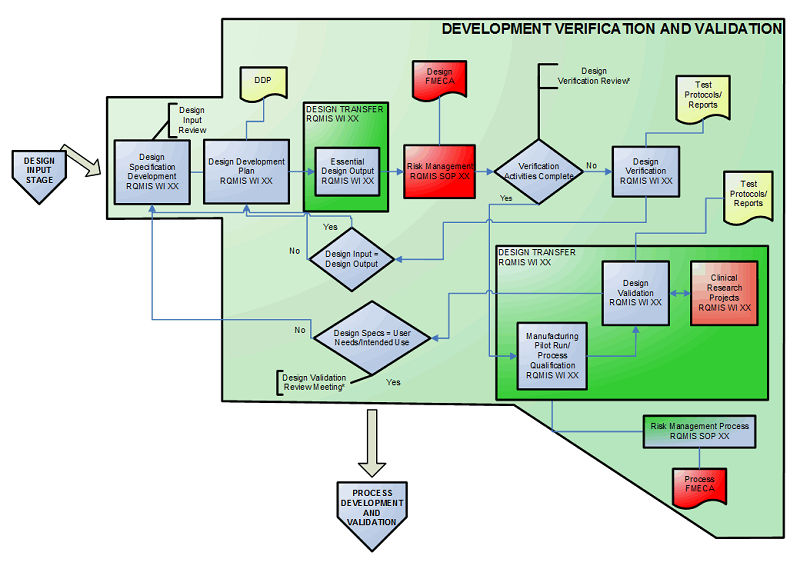
Design validation ensures that you designed the right device according to user needs the trap here is that design validation happens very late in the design process while defining user needs is something that happens near the beginning. Design validation is a design controls activity that happens pretty late in the product development process. Medical device validation rationale. Design validation is a testing process by which you prove validate that the device you ve built works for the end user as intended. The first four references are part of the definition of validation where the cmdr is referring to design validation.
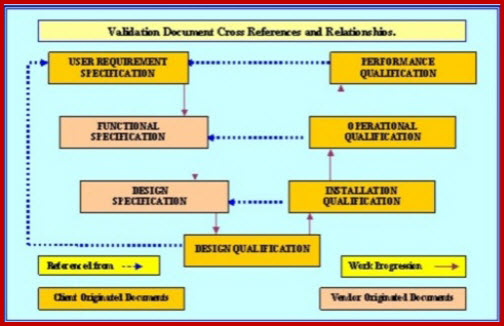
The purpose of design validation is to prove you designed the right device. For companies that hold one or more canadian medical device licenses validation appears in the canadian medical devices regulations cmdr a total of eight times four times as part of the french translation. There needs to be clear continuity throughout the overall design controls process to ensure a successful.
free printable late rent notice template free printable 1099 misc tax form template free privacy policy template for blog free letter of intent to purchase real estate template free printable kindergarten lesson plan template free one page business plan template free project manager resume template microsoft word